Dr. Vineet Choudhary
Present affiliation:
Email ID: vchoudhary@aiims.edu,
vineet.bps@gmail.com
Phone(O): +91-11-26594074 Lab Website:
Dr. Vineet Choudhary Research Interest:
Associate Professor,
Department of Biotechnology,
All India Institute of Medical Sciences (AIIMS),
Ansari Nagar,
New Delhi-110029
Current Research:
Molecular Mechanisms of cellular fat storage and degradation
Lipid droplets (LDs) are conserved dynamic intracellular organelles dedicated to fat storage. LDs play vital role in cell physiology and lipid metabolism.
Understanding LD biology is crucial to decipher the etiology of diseases such as obesity, atherosclerosis, and lipodystrophy (defects in homeostasis of fat tissue).
LDs originate from the endoplasmic reticulum (ER), and are surrounded by monolayer of phospholipids rather than a typical phospholipid bilayer membrane. The core of
droplets is composed of neutral lipids, such as triacylglycerols (TAG) and sterol esters (SE), which are produced by enzymes located in the ER. The LD surface harbors
structural proteins, such as perilipins, and lipid metabolic enzymes, including lipases and acyltransferases. Apart from their role in lipid homeostasis, LDs play role
in various cellular events, including protein degradation, ER stress response, they act as sites for assembly of infectious virions, are involved in membrane trafficking
and signal transduction, and act as a temporary storehouse of proteins such as transcription factors. Many fundamental aspects of cell biology of LDs remain poorly
characterized, such as how LD biogenesis, growth and regression is regulated, how protein trafficking between ER and LDs is facilitated, and how LDs establish close
contacts with other organelles, including vacuoles, peroxisomes, mitochondria, and the ER to achieve lipid homeostasis.
We have demonstrated that LDs do not spontaneously form at random locations in the ER, but rather originate at discrete ER subdomains. Marked by the protein seipin, these collaborate
with several LD biogenesis factors to establish proper assembly of droplet formation. Interestingly, seipin (Fld1 in yeast) is a non-enzymatic protein, lack of which results in
lipodystrophy syndromes, characterized by selective loss of adipose tissue. Seipin performs a decisive role in LD formation, lack of which results in LDs being born at ectopic
ER sites. The mechanism of how these specialized ER subdomains are formed is currently under investigation. We would like to uncover novel players that regulate LD formation at
pre-defined ER sites to identify potential therapeutic targets. Our studies will be performed in the model eukaryote, Saccharomyces cerevisiae and cultured mammalian cells using
an inter-disciplinary approach, combining genetics, biochemistry, cell biology, proteomics, fluorescence microscopy, and ultrastructural imaging using electron microscopy (EM).
We will use both in vivo and in vitro approach. Our findings in these basic processes would allow us to better understand what goes wrong in LD storage diseases in order to
develop therapeutics.
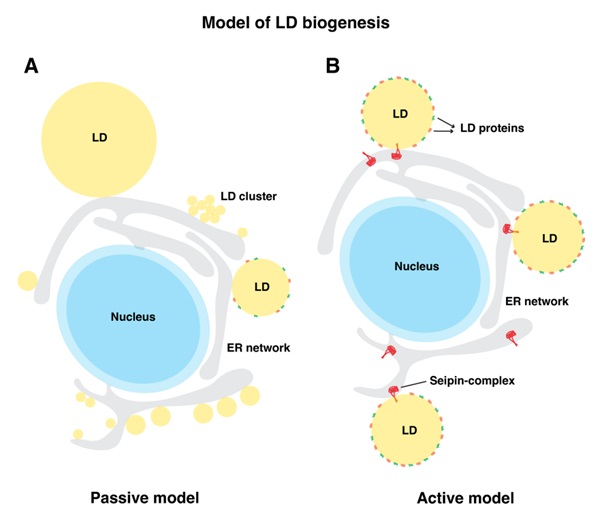
| A) Passive model: In a passive model of LD formation, neutral lipids are produced all over the ER in a random manner resulting in heterogeneous population of lipid droplets that exist either as numerous small or clustered LDs or a few large LDs. These stochastically formed LDs fail to enrich LD surface proteins. B) Active model: In the Active model, ER subdomains spatially defined by the seipin-complex recruits various LD-assembly factors and facilitates localized production of neutral lipids (NLs) and prevents its outflow into the ER membrane from these sites. At these sites lenses of NLs grow into nascent LDs. Seipin establishes functional ER-LD contacts that facilitates bi-directional transport of proteins and lipids between the two compartments. |
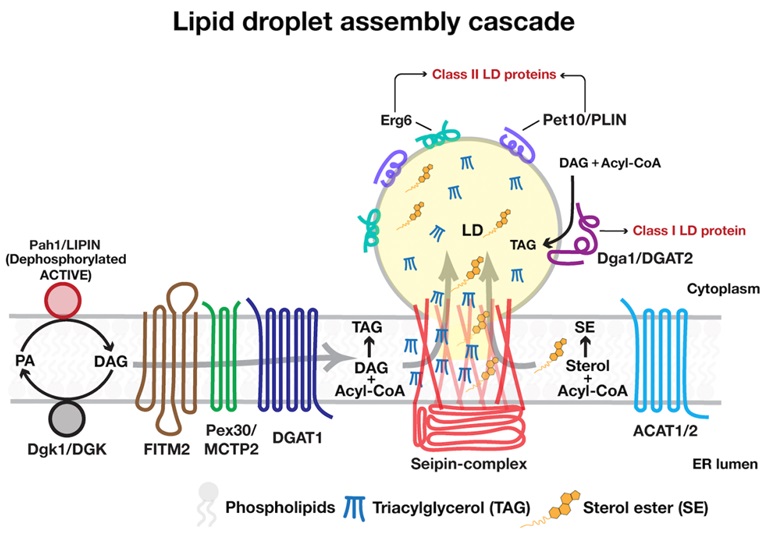
| Organization of LD biogenesis machinery Seipin-complex shows enrichment in tubular ER regions and establishes functional ER-LD contact site. Assembly of droplets begin with localized activation of the Lipin-complex that produces DAG. ER membrane proteins, FITM2 and Pex30 get recruited at these seipin marked ER subdomains. FITM2 binds to and regulates local DAG levels. Activity of Pex30 results in deformation of ER subdomains and remodeling of lipids at LD biogenesis sites to accommodate locally produced DAG and/or TAG. These lipids together with local membrane geometry at LD biogenesis sites facilitates recruitment of TAG-synthases, DGATs to catalyze NL production at these ER subdomains. Seipin-complex traps NLs within the hydrophobic helices and the transmembrane domain region prevents these NLs to diffuse into the bulk of the ER membrane thereby triggering nucleation of a nascent LD at these sites. These LDs acquire more NLs, emerge towards the cytoplasm and mature by acquiring class I and class II LD-resident proteins. Seipin-complex also plays a crucial role in the biogenesis of SE containing LDs. |
